Covalent Bonding in Atoms: Explained
Covalent bonding is one of the fundamental concepts in chemistry that explains how atoms share electrons to form molecules and compounds. It plays a crucial role in determining the structure, properties, and stability of substances found in nature and industrial applications.
Understanding covalent bonds helps explain why water flows, why diamonds are so strong, and how life itself is built from molecules like DNA and proteins. In this article, we will explore what covalent bonding is, how it works, its types, properties, and real-life applications.
What is Covalent Bonding?
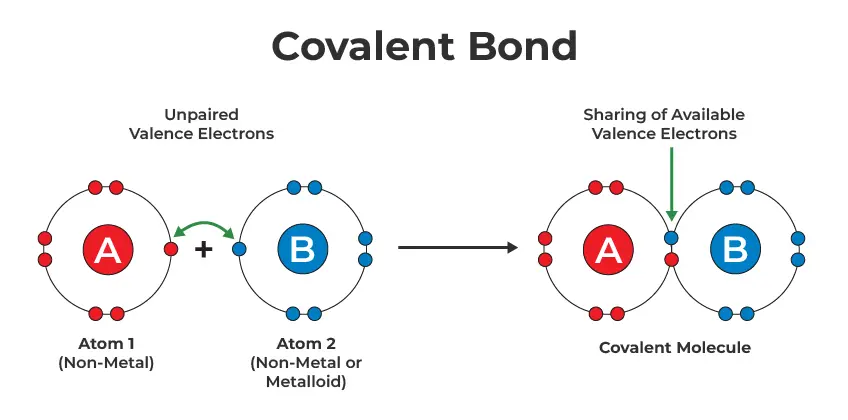
A covalent bond is a type of chemical bond where two atoms share one or more pairs of electrons to achieve stability. Unlike ionic bonds, where electrons are transferred, covalent bonds involve mutual sharing of electrons between atoms.
Why Do Atoms Form Covalent Bonds?
Atoms form covalent bonds to:
- Achieve a full outer electron shell (following the Octet Rule).
- Gain stability and lower their energy state.
- Form molecules and complex structures necessary for life and materials.
Covalent bonds are most commonly found in non-metal elements and molecular compounds.
Characteristics of Covalent Bonds
- Electron Sharing – Atoms share electrons instead of transferring them.
- Occurs Between Non-Metals – Typically found in non-metallic elements like oxygen, carbon, nitrogen, and hydrogen.
- Forms Stable Molecules – Covalent bonds create strong and stable molecular structures.
- Can Be Polar or Non-Polar – Based on electron distribution between atoms.
- Low Melting and Boiling Points – Compared to ionic compounds, most covalent compounds have lower melting and boiling points.
Types of Covalent Bonds
Covalent bonds can be classified based on the number of electron pairs shared between atoms.
1. Single Covalent Bond
- Involves one shared pair of electrons between two atoms.
- Example: Hydrogen (H₂), Chlorine (Cl₂), Methane (CH₄).
2. Double Covalent Bond
- Involves two shared pairs of electrons between atoms.
- Example: Oxygen (O₂), Carbon Dioxide (CO₂).
3. Triple Covalent Bond
- Involves three shared pairs of electrons, making it the strongest covalent bond.
- Example: Nitrogen (N₂), Acetylene (C₂H₂).
Polar vs. Non-Polar Covalent Bonds
Covalent bonds can be polar or non-polar, depending on the electronegativity of atoms involved.
1. Non-Polar Covalent Bond
- Electrons are shared equally between atoms.
- Occurs when atoms have similar electronegativities.
- Example: O₂, N₂, CH₄ (Molecules with symmetrical charge distribution).
2. Polar Covalent Bond
- Electrons are shared unequally, creating a partial charge (dipole moment).
- Occurs when one atom is more electronegative than the other.
- Example: Water (H₂O), where oxygen attracts electrons more than hydrogen, creating a slightly negative O and slightly positive H atoms.
Properties of Covalent Compounds
- Low Melting and Boiling Points – Most covalent compounds have weaker intermolecular forces than ionic compounds, leading to lower melting/boiling points.
- Poor Electrical Conductivity – Covalent compounds do not conduct electricity in solid or liquid form due to the absence of free-moving ions.
- Variable Solubility – Some covalent compounds dissolve in water (polar molecules like H₂O), while others dissolve in organic solvents (non-polar molecules like CH₄).
- Strong Intramolecular Forces – Covalent bonds within molecules are strong, but intermolecular forces (between molecules) can be weak.
- Exist in Different Physical States – Covalent compounds can be gases, liquids, or solids at room temperature.
Examples of Covalent Bonding in Everyday Life
Covalent bonds are present in various natural and synthetic materials that we use daily.
1. Water (H₂O)
- Formed by polar covalent bonds between hydrogen and oxygen atoms.
- Essential for life processes, hydration, and chemical reactions.
2. Carbon Dioxide (CO₂)
- Formed by double covalent bonds between carbon and oxygen.
- Plays a key role in photosynthesis, respiration, and the greenhouse effect.
3. Oxygen (O₂)
- A non-polar covalent molecule essential for breathing and combustion.
4. Diamond (C) and Graphite (C)
- Diamond: Each carbon forms four strong covalent bonds, making it the hardest known substance.
- Graphite: Carbon forms layered covalent bonds, allowing it to conduct electricity and be used as a lubricant.
5. DNA and Proteins
- Covalent bonds link atoms in DNA (phosphodiester bonds) and proteins (peptide bonds), forming the basic structure of life.
Comparison: Covalent Bonding vs. Ionic Bonding
| Feature | Covalent Bond | Ionic Bond |
|---|---|---|
| Electron Sharing/Transfer | Shared electrons | Transferred electrons |
| Occurs Between | Non-metals | Metals and non-metals |
| Strength | Strong but flexible | Strong and rigid |
| Melting/Boiling Point | Low to moderate | High |
| Conductivity | Poor conductor | Conducts in liquid state |
| Example | H₂O, CO₂, CH₄ | NaCl, KCl, MgO |
Applications of Covalent Bonding
Covalent bonds are crucial in various scientific and technological applications:
1. Medicine and Pharmaceuticals
- Used in drug molecules to ensure stability and targeted effects.
2. Materials Science
- Found in plastics, polymers, and synthetic fibers.
3. Electronics and Nanotechnology
- Graphene and carbon nanotubes are made of covalent bonds and used in high-tech applications.
4. Energy and Environment
- Methane (CH₄) and other hydrocarbons serve as fuel sources.
- Understanding CO₂ bonds helps in studying climate change.
5. Agriculture and Food Industry
- Proteins, carbohydrates, and vitamins rely on covalent bonds for structure and function.
Frequently Asked Questions (FAQs)
1. Why are covalent bonds strong?
Covalent bonds are strong due to electron sharing, which creates a stable balance of forces between atoms.
2. Can covalent compounds conduct electricity?
Most covalent compounds do not conduct electricity, except some polar covalent molecules in solution.
3. What is the strongest covalent bond?
Triple covalent bonds (e.g., N₂) are the strongest due to three shared electron pairs.
4. Why does water (H₂O) have a high boiling point despite being covalent?
Water has hydrogen bonding, a strong intermolecular force, which raises its boiling point.
5. Are diamonds covalent?
Yes, diamonds are purely covalent with a network of strong carbon-carbon bonds, making them extremely hard.
Conclusion
Covalent bonding is one of the most important types of chemical bonding, influencing biological processes, materials, and everyday substances. Whether it’s water, oxygen, DNA, or high-tech materials like graphene, covalent bonds shape the world around us.